Laboratory Testing Strategies by WHO for COVID-19
Covid-19 Molecular/PCR tests
Have Any Questions?
If you have any query or looking for the best medical laboratory then Bio Covid Lab is always there. feel free to Contact.
What is COVID-19?
Coronavirus Disease 2019 (COVID-19) is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). COVID-19 was identified in December 2019 and declared a global pandemic by the World Health Organization in March 2020.
Type and severity of symptoms of an active COVID-19 infection vary greatly between individuals depending on the age, gender, and underlying medical conditions. The most commonly reported ones include fever, loss of smell, coughing, sore throat, shortness of breath or difficulty breathing, feeling weak or lethargic, chills, muscle pain, light-headedness or dizziness, headache, vomiting or diarrhea, slurred speech, and/or seizures. Less common symptoms include stuffy nose, conjunctivitis (pink eye), dizziness, confusion, abdominal pain, and skin rashes or discoloration of fingers or toes.
Type and severity of symptoms of an active COVID-19 infection vary greatly between individuals depending on the age, gender, and underlying medical conditions. The most commonly reported ones include fever, loss of smell, coughing, sore throat, shortness of breath or difficulty breathing, feeling weak or lethargic, chills, muscle pain, light-headedness or dizziness, headache, vomiting or diarrhea, slurred speech, and/or seizures. Less common symptoms include stuffy nose, conjunctivitis (pink eye), dizziness, confusion, abdominal pain, and skin rashes or discoloration of fingers or toes.
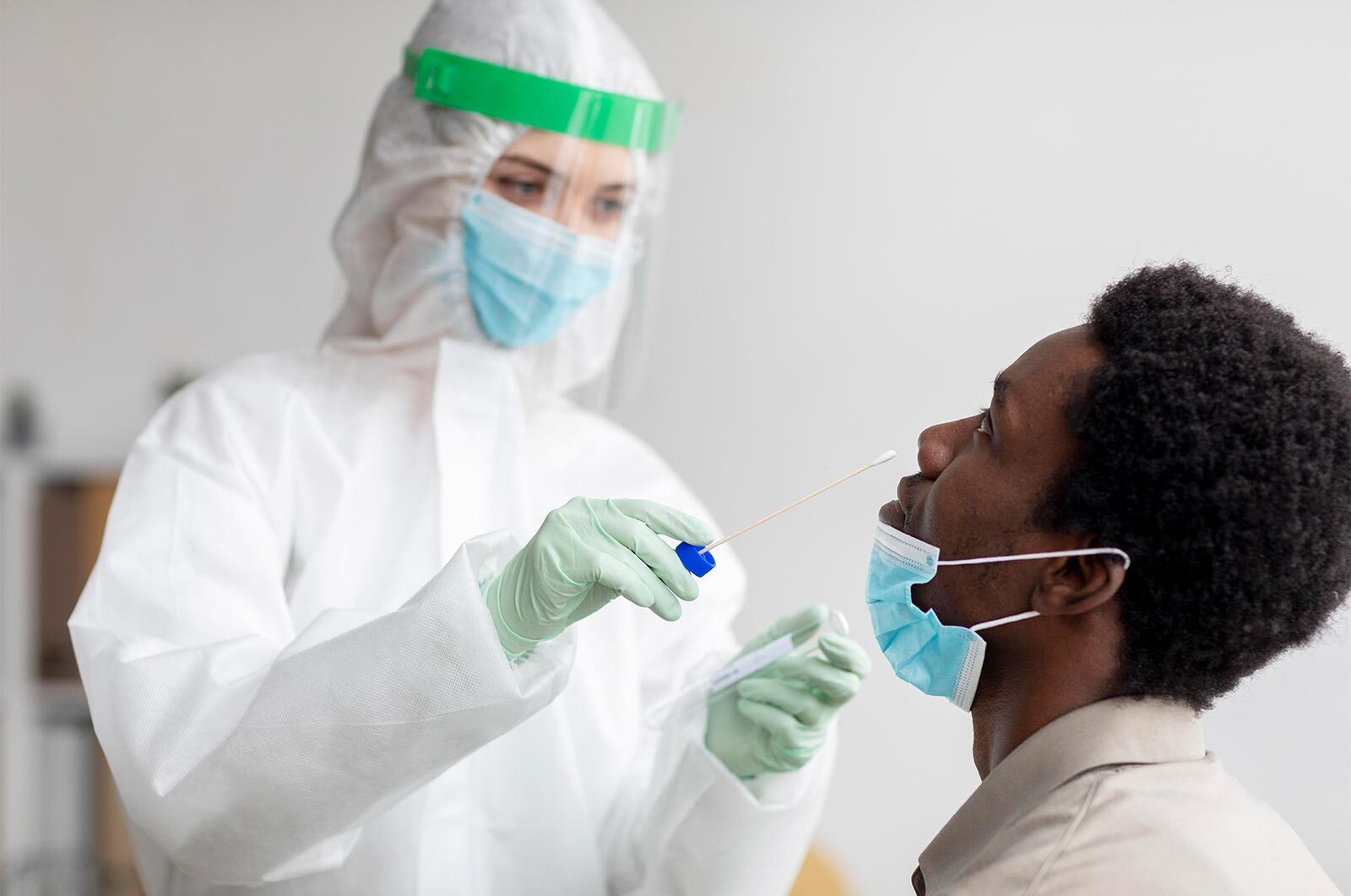
Molecular/PCR Test
Molecular tests detect genetic material – the RNA – of the coronavirus and are sensitive enough to need only a very tiny amount of it.
The sample is collected with a nasal or throat swab and they tend to take hours to provide results.
Procedure Of Molecular/PCR Test
Specimen: Nasopharyngeal / oropharyngeal Swab
What the test will look for: Nucleic acids ie. RNA of Covid-19 Virus.
Process: A sample is collected from deep inside the patient’s nose and the back of the throat with a swab. The sample is then treated with chemical solutions that remove proteins and fats, leaving only the RNA present in the sample. The sample is then sent to the laboratory for analysis where an RT-PCR machine is used to detect the virus. This is a molecular test which looks for the genetic material of the virus. Real Time-PCR test is intended for the qualitative detection of nucleic acid from the 2019-nCoV in upper and lower respiratory specimens collected from individuals who meet 2019-nCoV clinical or epidemiological criteria.
What the test will look for: Nucleic acids ie. RNA of Covid-19 Virus.
Process: A sample is collected from deep inside the patient’s nose and the back of the throat with a swab. The sample is then treated with chemical solutions that remove proteins and fats, leaving only the RNA present in the sample. The sample is then sent to the laboratory for analysis where an RT-PCR machine is used to detect the virus. This is a molecular test which looks for the genetic material of the virus. Real Time-PCR test is intended for the qualitative detection of nucleic acid from the 2019-nCoV in upper and lower respiratory specimens collected from individuals who meet 2019-nCoV clinical or epidemiological criteria.
Difference between molecular (PCR) test and Antibody test
The molecular test (polymerase chain reaction (PCR)):
detects SARS-CoV-2 genetic material and is used to diagnose an active COVID-19 infection. A sample type used in molecular testing is a nasopharyngeal (NP) swab.
The COVID-19 antibody test:
is a blood test to detect antibodies to the virus. Results of the test show whether you had a recent or prior COVID-19 infection. The antibody test is not to be used for diagnosis of active COVID-19 infection.

Questions about test results
- Which type of molecular test did I have, and how accurate is that testing method?
- Based on my test result, do I need to take any special precautions related to COVID-19?
- Is there any benefit to repeating the test?
- Are there other types of tests that might be helpful in my situation?
